is vegetable oil polar or nonpolar
The wax dissolved in the oil. Acetone is also relatively safe organic solvent but you should still keep it away from fire and wear your mask and gloves.

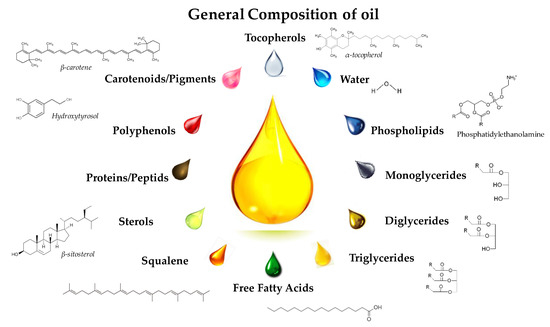
Molecules Free Full Text Vegetable Oils As Alternative Solvents For Green Oleo Extraction Purification And Formulation Of Food And Natural Products Html
The hydrocarbon tails of soap are nonpolar so they are soluble in oil.
. Get help with your Solubility homework. The nonpolar sections wins out and determines its solubility in water. There is a net transfer of one or more species from one liquid into another liquid phase generally from aqueous to. The hydrocarbon tails of soap are polar so they are soluble in oil.
Polar things that can be mixed into water like sugar and nonpolar things that cannot be mixed into water like oil. The InvisiCrepe Body Balm formula is claimed to make long-term repairs to the skin barrier and help strengthen the skins cellular protein matrix resulting in a smoother. Place equal amounts of the substance into each beaker. In this example most of the molecule is nonpolar the long C-H tail with just a small part at the right end being polar.
Now take a length of thread or a long. Oleic acid found in olive oil is soluble in hexane but not soluble in water. When the soapy water is rinsed away the trapped grease and oil is washed away with it. For example you could put vegetable oil into the second container.
This was followed by adding about a tablespoon of vegetable oil and mixing with the spoon. Put the substance in question into the. It is not volatile but still acts as a nonpolar solvent. Nonpolar Tails Polar Head Phosphatidyl Choline Lecithin 44 Lecithin solid ethanol petroleum ether glycerol stearic acid oleic acid vegetable oil 1 starch solution in water 05 iodine solution in petroleum ether food samples for extraction of lipids test tubes 250 mL Erlenmeyer flask and 50 mL or 100 mL beaker.
Which of the following is the best explanation for why soaps can dissolve oily substances. Vegetable oil concentrates have performed less consistently than their petroleum-based counterparts. Polar mediums such as water allow changes in the phenolic compound levels. In addition City Beauty is cruelty-free and does not test on animals.
Acetone is a volatile compound. When a soap micelle encounters oil or grease these non-polar materials are forced to the inside of the micelle to get away from the polar water and polar heads of the micelle where they are trapped. This gives soap the ability to dissolve most types of molecules making it. All the ions and proteins in a cell are dissolved in water within the cell.
Oil and water dont mix because water has polar bonds while oils have nonpolar bonds. A quick chemistry refresher will remind us that there are two general types of molecules. A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. Solubility of Lipids Add about 01 g of.
Solubility Questions and Answers. Low-temperature water extraction could obtain more water-soluble substances while high-temperature water extraction could extract less soluble substances. It formed a separate layers in water. Pour some water into a shallow bowl.
However manufacturers are attempting to improve plant or vegetable-based oils by increasing their nonpolar or lipophilic characteristics. It occurred to me that ordinary vegetable oil is a cheap and of course innocuous lipophilic substance. I spilled about 2 oz molten candle wax on a marble surface and after it solidified removed a good portion by scraping with a plastic spoon. For this reason it is possible to extract non-polar moderately polar and polar chemical compounds.
In contrast if the medium is nonpolar use of oil for frying in both deep frying and pan frying the loss of compounds is lower due to the lack of diffusion or migration to the medium 19. Access the answers to hundreds of Solubility questions that are explained in a way thats easy for you to understand. The most common method has been through esterification of common seed oils such as methylated sunflower soybean cotton. Soap molecules are amphipathic meaning they have both polar and non-polar properties.
Liquidliquid extraction LLE also known as solvent extraction and partitioning is a method to separate compounds or metal complexes based on their relative solubilities in two different immiscible liquids usually water polar and an organic solvent non-polar. InvisiCrepe Body Balm is formulated without parabens sulfates phthalates mineral oil gluten nuts and animal-derived ingredients. Furthermore liquid water at elevated temperature is a solvent of lower polarizabilitypolarity.

Is Vegetable Oil A Polar Compound Quora

Molecules Free Full Text Vegetable Oils As Alternative Solvents For Green Oleo Extraction Purification And Formulation Of Food And Natural Products Html

Is Vegetable Oil A Polar Compound Quora

Molecules Free Full Text Vegetable Oils As Alternative Solvents For Green Oleo Extraction Purification And Formulation Of Food And Natural Products Html

Molecules Free Full Text Vegetable Oils As Alternative Solvents For Green Oleo Extraction Purification And Formulation Of Food And Natural Products Html
Posting Komentar untuk "is vegetable oil polar or nonpolar"